Electron
 |
|
| Composition: | Elementary particle[2] |
| Particle statistics: | Fermionic |
| Group: | Lepton |
| Generation: | First |
| Interaction: | Gravity, Electromagnetic, Weak |
| Symbol(s): | e− , β− |
| Antiparticle: | Positron (also called antielectron) |
| Theorized: | Richard Laming (1838–1851),[3] G. Johnstone Stoney (1874) and others.[4][5] |
| Discovered: | J. J. Thomson (1897)[6] |
| Mass: | 9.10938215(45)×10−31 kg[7] 5.4857990943(23)×10−4 u[7] [1,822.88850204(77)]−1 u[note 1] 0.510998910(13) MeV/c2[7] |
| Electric charge: | −1 e[note 2] −1.602176487(40)×10−19 C[7] −4.803×10−10 esu [8] |
| Magnetic moment: | −1.00115965218111 μB[7] |
| Spin: | 1⁄2 |
The electron is a subatomic particle carrying a negative electric charge. It has no known components or substructure. Therefore, the electron is generally believed to be an elementary particle.[2] An electron has a mass that is approximately 1/1836 that of the proton[9] The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion. The antiparticle of the electron is called the positron. The positron is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may either scatter off each other or be totally annihilated, producing a pair (or more) of gamma ray photons. Electrons, which belong to the first generation of the lepton particle family,[10] participate in gravitational, electromagnetic and weak interactions.[11] Electrons, like all matter, have quantum mechanical properties of both particles and waves, so they can collide with other particles and be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[10]
The concept of an indivisible amount of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopher Richard Laming;[4] the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.[6][12][13]
In many physical phenomena, such as electricity, magnetism, and thermal conductivity, electrons play an essential role. An electron in motion relative to an observer generates a magnetic field, and will be deflected by external magnetic fields. When an electron is accelerated, it can absorb or radiate energy in the form of photons. Electrons, together with atomic nuclei made of protons and neutrons, make up atoms. However, electrons contribute less than 0.06% to an atom's total mass. The attractive Coulomb force between an electron and a proton causes electrons to be bound into atoms. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[14]
According to theory, most electrons in the universe were created in the big bang, but they may also be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. Electrons may be destroyed through annihilation with positrons, and may be absorbed during nucleosynthesis in stars. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including welding, cathode ray tubes, electron microscopes, radiation therapy, lasers and particle accelerators.
Contents[hide] |
History
The ancient Greeks noticed that amber attracted small objects when rubbed with fur. Apart from lightning, this phenomenon is humanity's earliest recorded experience with electricity.[15] In his 1600 treatise De Magnete, the English scientist William Gilbert coined the New Latin term electricus, to refer to this property of attracting small objects after being rubbed.[16] Both electric and electricity are derived from the Latin ēlectrum (also the root of the alloy of the same name), which came from the Greek word ήλεκτρον (ēlektron) for amber.
In 1737 C. F. du Fay and Hawksbee independently discovered what they believed to be two kinds of frictional electricity; one generated from rubbing glass, the other from rubbing resin. From this, Du Fay theorized that electricity consists of two electrical fluids, "vitreous" and "resinous", that are separated by friction and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought that the charge carrier was positive.[19]
Between 1838 and 1851, British natural philosopher Richard Laming developed the idea that an atom is composed of a core of matter surrounded by subatomic particles that had unit electric charges.[3] Beginning in 1846, German physicist William Weber theorized that electricity was composed of positively and negatively charged fluids, and their interaction was governed by the inverse square law. After studying the phenomenon of electrolysis in 1874, Irish physicist George Johnstone Stoney suggested that there existed a "single definite quantity of electricity", the charge of a monovalent ion. He was able to estimate the value of this elementary charge e by means of Faraday's laws of electrolysis.[20] However, Stoney believed these charges were permanently attached to atoms and could not be removed. In 1881, German physicist Hermann von Helmholtz argued that both positive and negative charges were divided into elementary parts, each of which "behaves like atoms of electricity".[4]
In 1894, Stoney coined the term electron to describe these elementary charges, saying, "... an estimate was made of the actual amount of this most remarkable fundamental unit of electricity, for which I have since ventured to suggest the name electron".[21] The word electron is a combination of the word electric and the suffix -on, with the latter now used to designate a subatomic particle, such as a proton or neutron.[22][23]
Discovery

The German physicist Johann Wilhelm Hittorf undertook the study of electrical conductivity in rarefied gases. In 1869, he discovered a glow emitted from the cathode that increased in size with decrease in gas pressure. In 1876, the German physicist Eugen Goldstein showed that the rays from this glow cast a shadow, and he dubbed the rays cathode rays.[25] During the 1870s, the English chemist and physicist Sir William Crookes developed the first cathode ray tube to have a high vacuum inside.[26] He then showed that the luminescence rays appearing within the tube carried energy and moved from the cathode to the anode. Furthermore, by applying a magnetic field, he was able to deflect the rays, thereby demonstrating that the beam behaved as though it were negatively charged.[27][28] In 1879, he proposed that these properties could be explained by what he termed 'radiant matter'. He suggested that this was a fourth state of matter, consisting of negatively charged molecules that were being projected with high velocity from the cathode.[29]
The German-born British physicist Arthur Schuster expanded upon Crookes' experiments by placing metal plates in parallel to the cathode rays and applying an electric potential between the plates. The field deflected the rays toward the positively charged plate, providing further evidence that the rays carried negative charge. By measuring the amount of deflection for a given level of current, in 1890 Schuster was able to estimate the charge-to-mass ratio of the ray components. However, this produced a value that was more than a thousand times greater than what was expected, so little credence was given to his calculations at the time.[27][30]
In 1896, the British physicist J. J. Thomson, with his colleagues John S. Townsend and H. A. Wilson,[12] performed experiments indicating that cathode rays really were unique particles, rather than waves, atoms or molecules as was believed earlier.[6] Thomson made good estimates of both the charge e and the mass m, finding that cathode ray particles, which he called "corpuscles," had perhaps one thousandth of the mass of the least massive ion known: hydrogen.[6][13] He showed that their charge to mass ratio, e/m, was independent of cathode material. He further showed that the negatively charged particles produced by radioactive materials, by heated materials and by illuminated materials were universal.[6][31] The name electron was again proposed for these particles by the Irish physicist George F. Fitzgerald, and the name has since gained universal acceptance.[27]
While studying naturally fluorescing minerals in 1896, the French physicist Henri Becquerel discovered that they emitted radiation without any exposure to an external energy source. These radioactive materials became the subject of much interest by scientists, including the New Zealand physicist Ernest Rutherford who discovered they emitted particles. He designated these particles alpha and beta, on the basis of their ability to penetrate matter.[32] In 1900, Becquerel showed that the beta rays emitted by radium could be deflected by an electric field, and that their mass-to-charge ratio was the same as for cathode rays.[33] This evidence strengthened the view that electrons existed as components of atoms.[34][35]
The electron's charge was more carefully measured by the American physicist Robert Millikan in his oil-drop experiment of 1909, the results of which he published in 1911. This experiment used an electric field to prevent a charged droplet of oil from falling as a result of gravity. This device could measure the electric charge from as few as 1–150 ions with an error margin of less than 0.3%. Comparable experiments had been done earlier by Thomson's team,[6] using clouds of charged water droplets generated by electrolysis,[12] and in 1911 by Abram Ioffe, who independently obtained the same result as Millikan using charged microparticles of metals, then published his results in 1913.[36] However, oil drops were more stable than water drops because of their slower evaporation rate, and thus more suited to precise experimentation over longer periods of time.[37]
Around the beginning of the twentieth century, it was found that under certain conditions a fast moving charged particle caused a condensation of supersaturated water vapor along its path. In 1911, Charles Wilson used this principle to devise his cloud chamber, allowing the tracks of charged particles, such as fast-moving electrons, to be photographed.[38]
Atomic theory
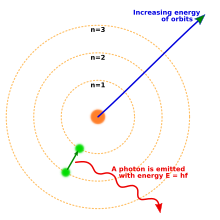
By 1914, experiments by physicists Ernest Rutherford, Henry Moseley, James Franck and Gustav Hertz had largely established the structure of an atom as a dense nucleus of positive charge surrounded by lower-mass electrons.[39] In 1913, Danish physicist Niels Bohr postulated that electrons resided in quantized energy states, with the energy determined by the angular momentum of the electron's orbits about the nucleus. The electrons could move between these states, or orbits, by the emission or absorption of photons at specific frequencies. By means of these quantized orbits, he accurately explained the spectral lines of the hydrogen atom.[40] However, Bohr's model failed to account for the relative intensities of the spectral lines and it was unsuccessful in explaining the spectra of more complex atoms.[39]
Chemical bonds between atoms were explained by Gilbert Newton Lewis, who in 1916 proposed that a covalent bond between two atoms is maintained by a pair of electrons shared between them.[41] Later, in 1923, Walter Heitler and Fritz London gave the full explanation of the electron-pair formation and chemical bonding in terms of quantum mechanics.[42] In 1919, the American chemist Irving Langmuir elaborated on the Lewis' static model of the atom and suggested that all electrons were distributed in successive "concentric (nearly) spherical shells, all of equal thickness".[43] The shells were, in turn, divided by him in a number of cells each containing one pair of electrons. With this model Langmuir was able to qualitatively explain the chemical properties of all elements in the periodic table,[42] which were known to largely repeat themselves according to the periodic law.[44]
In 1924, Austrian physicist Wolfgang Pauli observed that the shell-like structure of the atom could be explained by a set of four parameters that defined every quantum energy state, as long as each state was inhabited by no more than a single electron. (This prohibition against more than one electron occupying the same quantum energy state became known as the Pauli exclusion principle.)[45] The physical mechanism to explain the fourth parameter, which had two distinct possible values, was provided by the Dutch physicists Abraham Goudsmith and George Uhlenbeck when they suggested that an electron, in addition to the angular momentum of its orbit, could possess an intrinsic angular momentum.[39][46] This property became known as spin, and explained the previously mysterious splitting of spectral lines observed with a high-resolution spectrograph; this phenomenon is known as fine structure splitting.[47]
Quantum mechanics
In his 1924 dissertation Recherches sur la théorie des quanta (Research on Quantum Theory), French physicist Louis de Broglie hypothesized that all matter possesses a De Broglie wave similar to light.[48] That is, under the appropriate conditions, electrons and other matter would show properties of either particles or waves. The corpuscular properties of a particle are demonstrated when it is shown to have a localized position in space along its trajectory at any given moment.[49] Wave-like nature is observed, for example, when a beam of light is passed through parallel slits and creates interference patterns. In 1927, the interference effect was demonstrated with a beam of electrons by English physicist George Paget Thomson with a thin metal film and by American physicists Clinton Davisson and Lester Germer using a crystal of nickel.[50]

The success of de Broglie's prediction led to the publication, by Erwin Schrödinger in 1926, of the Schrödinger equation that successfully describes how electron waves propagated.[51] Rather than yielding a solution that determines the location of an electron over time, this wave equation can be used to predict the probability of finding an electron near a position. This approach was later called quantum mechanics, which provided an extremely close derivation to the energy states of an electron in a hydrogen atom.[52] Once spin and the interaction between multiple electrons were considered, quantum mechanics allowed the configuration of electrons in atoms with higher atomic numbers than hydrogen to be successfully predicted.[53]
In 1928, building on Wolfgang Pauli's work, Paul Dirac produced a model of the electron - the Dirac equation, consistent with relativity theory, by applying relativistic and symmetry considerations to the hamiltonian formulation of the quantum mechanics of the electro-magnetic field.[54] In order to resolve some problems within his relativistic equation, in 1930 Dirac developed a model of the vacuum as an infinite sea of particles having negative energy, which was dubbed the Dirac sea. This led him to predict the existence of a positron, the antimatter counterpart of the electron.[55] This particle was discovered in 1932 by Carl D. Anderson, who proposed calling standard electrons negatrons, and using electron as a generic term to describe both the positively and negatively charged variants. This usage of the term 'negatron' is still occasionally encountered today, and it may be shortened to 'negaton'.[56][57]
In 1947 Willis Lamb, working in collaboration with graduate student Robert Rutherford, found that certain quantum states of hydrogen atom, which should have the same energy, were shifted in relation to each other, the difference being the Lamb shift. About the same time, Polykarp Kusch, working with Henry M. Foley, discovered the magnetic moment of the electron is slightly larger than predicted by Dirac's theory. This small difference was later called anomalous magnetic dipole moment of the electron. To resolve these issues, a refined theory called quantum electrodynamics was developed by Sin-Itiro Tomonaga, Julian Schwinger and Richard P. Feynman in the late 1940s.[58]
Particle accelerators
With the development of the particle accelerator during the first half of the twentieth century, physicists began to delve deeper into the properties of subatomic particles.[59] The first successful attempt to accelerate electrons using Electromagnetic induction was made in 1942 by Donald Kerst. His initial betatron reached energies of 2.3 MeV, while subsequent betatrons achieved 300 MeV. In 1947, synchrotron radiation was discovered with a 70 MeV electron synchrotron at General Electric. This radiation was caused by the acceleration of electrons, moving near the speed of light, through a magnetic field.[60]
With a beam energy of 1.5 GeV, the first high-energy particle collider was ADONE, which began operations in 1968.[61] This device accelerated electrons and positrons in opposite directions, effectively doubling the energy of their collision when compared to striking a static target with an electron.[62] The Large Electron-Positron Collider (LEP) at CERN, which was operational from 1989 to 2000, achieved collision energies of 209 GeV and made important measurements for the Standard Model of particle physics.[63][64]
Characteristics
Classification
In the Standard Model of particle physics, electrons belong to the group of subatomic particles called leptons, which are believed to be fundamental or elementary particles. Electrons have the lowest mass of any charged lepton (or electrically charged particle of any type) and belong to the first-generation of fundamental particles.[65] The second and third generation contain charged leptons, the muon and the tau, which are identical to the electron in charge, spin and interactions, but are more massive. Leptons differ from the other basic constituent of matter, the quarks, by their lack of strong interaction. All members of the lepton group are fermions, because they all have half-odd integer spin; the electron has spin 1⁄2.[66]
Fundamental properties
The invariant mass of an electron is approximately 9.109×10−31
نظرات شما عزیزان:
kilogram,[7] or 5.489×10−4
atomic mass unit. On the basis of Einstein's principle of mass–energy equivalence, this mass corresponds to a rest energy of 0.511 MeV. The ratio between the mass of a proton and that of an electron is about 1836.[9]